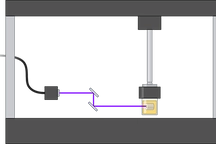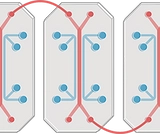
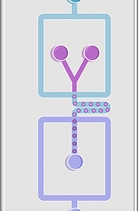
Microfabrication and OOC
Organ-on-a-chip (OoC) platforms seek to recapitulate certain aspects of human organ physiology at a microscopic scale by integrating microfluidics and three-dimensional (3D) models of human tissues. OoCs offer a vast number of applications in almost every aspect of the human anatomy. For instance, OoCs will be able to provide accurate and reliable predictions of the effects of drugs and toxins on human bodies in the near future. Nowadays, we are working on developing personalized physiological models that we hope will be used as a versatile platform to develop more precise medical therapies. Our research efforts are also focused on observing the essential functions of cells and the interactions between the input and output, which will also be crucial for reducing the experimental work on animal models, alined with the principles of the 3Rs (Replacement, Reduction and Refinement).
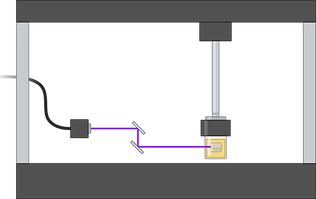


Additive manufacturing, 3D bioprinting and XR technologies
3D bioprinting is an
emerging
technology that can spatially control the construction
process of engineered tissues. The BTELab focuses on controlling the printed constructs'
architecture, morphology, and biological properties to generate 3D platforms that reproduce
their
human equivalents fairly. Our latest works show an improvement in this technology for different
aspects, such as greater functionality of the printed tissues through better vascularization and
exploring the synergy with other additive manufacturing techniques. Our team's
multidisciplinarity
enables the control of the process from the early stage of machine design to cell preparation,
which
allows us to adapt the 3D printers to the specific needs of different projects and research
lines.
The main applications of our bioprinted constructs in the lab are the following:
- Study of tissue and organ regeneration
- Explore mechanisms of diseases
- Drug sensitivity tests
- Drug development
We are developing broad collaborations with various stakeholders to investigate other potential avenues of investigation and treatment.
3D PRINTING IN CLINICS
We are interested in applying 3D printing techniques in clinical contexts to create realistic and anatomically precise 3D printed models generated from medical images for surgery planning, training, and teaching applications. Together with other medical departments and hospital services, we have started a pioneer work to provide them with these complex and personalized 3D realistic models that can potentially be used in their surgeries or teaching activities. BTELab facilities include the most advanced technologies to create the models in different polymers and resins, controlling not only colors and textures but also stiffness and elongation. Our expertise in medical image processing and digital model manipulation is essential to be capable of extracting the whole complexity and anatomical structure of the human organs and tissues from the medical images.
XR TECHNOLOGIES
Our research is also dedicated to creating extended reality (XR) environments for manipulating personalized patient anatomical models, facilitating complex surgical planning and medical education. We're developing virtual reality (VR) and augmented reality (AR) systems to enrich surgical teaching methods. Our current focus is on integrating advanced haptic feedback through mixed reality (MR) phantoms, pushing the boundaries of immersive medical training.
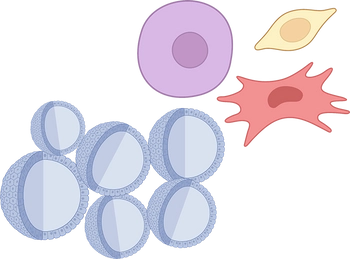
Stem cell biology and Tissue Engineering
Stem cells can be self-replicating and differentiate into different cell types depending on their potency. Our group studies stem cell-based treatments and develop stem cell delivery technologies with or without the help of scaffolds mimicking the properties of the natural niche. We use human multipotent adipose mesenchymal stem cells (hASCs) as a clinically relevant source for tissue engineering but also as a model system for differentiation and signal transduction. We prepare 3D in vitro platforms using hASCs, spheroids, and organoids. Research activities include immunomodulation and wound healing. Creating models and substitutes using engineering approaches and xeno-free cell derivatives, designing directed differentiation protocols. Our efforts are also looking to identify the factors central to mediated regulation of angiogenesis, fibrosis, immune modulation, and epigenetics. We have a library of hASCs from over 200 different donors fully characterized and tested against pathogens, adventitious viruses, or zoonotic agents.